___
WHO to new PCR users: read the damned manual!
January 23, 2021 | Author Ian M Mackay, PhD (EIC)The World Health Organization (WHO) recently published (14th Dec 2020, then updated 20th Jan 2021) a piece of advice for laboratories testing for SARS-CoV-2 using PCR. The advice boils down: new to PCR? Read the instructions and understand the purpose of testing. But of course, this lab-focussed advice has been taken by those with malicious intent, or with too little understanding of the topic, and blown it up into something else entirely wrong.
Target audience probably isn’t you
Look, the internet doesn’t necessarily always write just for you.
I’m sorry because this will come as a shock to some. It will disappoint others. A few will currently be pouring gasoline over their phone and lighting it on fire.
But as the WHO went to some extra lengths to make clear…
Target audience: laboratory professionals and users of IVDs.
From the people who wrote the document
That means for most of the planet – there’s nothing to see here because this is literally a document that says – “make sure you’ve read the manual”. It’s not even directed at expert pathology labs who already know what they are doing.
If you’re in the target audience – listen up
This was written because some of you, perhaps some doing high-throughput testing of human specimens for the first time ever – need to take some time to learn about what it is you’re trying to achieve here.
Basically, the document specifies that a PCR result is one part of a process of diagnosis. It’s to be taken in context. I feel like I may have said this 2,765 times before:
Most PCR assays are indicated as an aid for diagnosis, therefore, health care providers must consider any result in combination with timing of sampling, specimen type, assay specifics, clinical observations, patient history, confirmed status of any contacts, and epidemiological information..
From [1], bolding by me.
If you don’t use or run PCR in a pathology laboratory setting….
…you might need to go delete that tweet or post because you could be very wrong in your interpretation of this notice from the WHO.
Some certainly have posted material that was unsupported or wrong – and it was pounced upon by those among the angry anti-PCR (and anti-other things) cult.
Some examples
The misunderstanding below is around the WHO’s comment not to mess with the threshold for determining the threshold cycle (CT), if that contravenes the real-time RT-PCR test kit’s instructions for use (IFU) and you have no experience with doing so.
I have no idea how that was misinterpreted into becoming about changing the number of cycles used the RT-rPCR though. This bold but wrong statement was quickly retweeted (now deleted thankfully) and spread far and wide (the account has 330,768 followers and a television audience).
When a topic is so far removed from your area of expertise, it can be best to make doubly sure that you choose your words carefully. Your influence matters.
Another tweeter seems to be revelling in the WHO notice saying something more than it does as well, enthusiastically claiming they were right all along about their (actually quite wrong) claims of PCR test generating false positives. Sadly for them, the WHO notice provides no such support.
In the example below, the tweeter seems to have fallen into the same trap of misunderstanding, misreading or not reading at all the WHO Information Notice. They seem to think it says that the cycle number could be reduced. The Notice doesn’t say this.

Also, even if you were to reduce the cycle number by 10 (from, for example, 45 to 35), for the sake of appeasement, the majority of positives would still be uncontroversially positive if we go by the UK testing data below.

In this headline, the writer seems to have added in something about PCR not identifying a case of COVID-19 and a second test being required according to the WHO Notice.

Context is really lacking here. The WHO Notice doesn’t state this as written and this will likely lead some who only read headlines to walk away with the wrong impression.
WHO guidance Diagnostic testing for SARS-CoV-2 states that careful interpretation of weak positive results is needed (1). The cycle threshold (Ct) needed to detect virus is inversely proportional to the patient’s viral load. Where test results do not correspond with the clinical presentation, a new specimen should be taken and retested using the same or different NAT technology.
Quote from the WHO Notice [1]
The Notice discusses weak positive results (late or high CTs) – not all positives. It goes on to say that if the test doesn’t fit with the patient’s health (I’d add contact and epidemiological history) status, a new sample should be collected and the test repeated. A lab may use the initial test or a different test (good labs are armed with tests that target more than one genetic region of SARS-CoV-2 or come from more than one commercial supplier).
I could go on with examples and showing you how they are flawed takes – for whatever reason(s) – but you probably get the picture now.
The WHO Notice was clear, basic and meant for those labs that need some mentorship in pathology testing.
References
- WHO Information Notice for IVD Users 2020/05
https://www.who.int/news/item/20-01-2021-who-information-notice-for-ivd-users-2020-05 - Coronavirus (COVID-19) Infection Survey, UK: 22 January 2021
https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveypilot/22january2021 - WHO releases new tightened guidelines regarding the diagnostic criteria for COVID-19. PCR positive no longer means you have COVID-19. You need a second test to confirm you have the virus
https://worldnewsera.com/news/startups/who-releases-new-tightened-guidelines-regarding-the-diagnostic-criteria-for-covid-19-pcr-positive-no-longer-means-you-have-covid-19-you-need-a-second-test-to-confirm-you-have-the-virus-tech-news/
___
5 thoughts on “WHO to new PCR users: read the damned manual!”
___
Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020
Since the emergence of coronavirus disease (COVID-19) at the end of 2019, rapid tracing and isolation of confirmed cases and close contacts with restrictions on social movement have played an important role in controlling onward spread of the virus. Understanding the duration of infectiousness in persons who test positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is critical to developing evidence-based public health policies on isolation, contact tracing and return to work. Virus detection by reverse transcription-PCR (RT-PCR) from respiratory samples is widely used to diagnose and monitor SARS-CoV-2 infection and, increasingly, to infer infectivity of an individual. However, RT-PCR does not distinguish between infectious and non-infectious virus. Propagating virus from clinical samples confirms the presence of infectious virus but is not widely available, requires biosafety level 3 facilities, and the results are not timely to inform public health actions. The aim of this work was to understand how RT-PCR detection relates to cultivable virus, which can be used as a proxy for infectiousness and can inform and support decisions on infection control.
Upper respiratory tract (URT) samples from persons with suspected COVID-19 were tested at the national respiratory virus reference laboratory at Public Health England to support routine clinical care and surveillance activities during the COVID-19 pandemic. Samples included nose, throat, combined nose-and-throat and nasopharyngeal swabs, or nasopharyngeal aspirates; the majority were taken by clinical staff but some were self-sampled nose swabs.
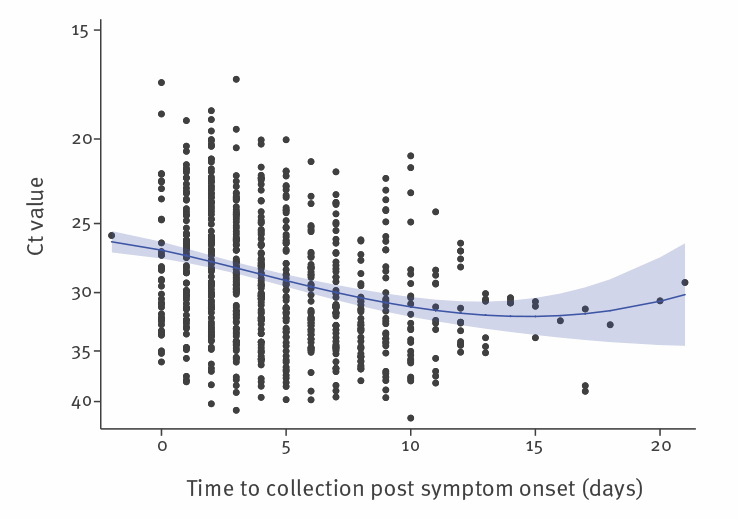
In the first 3 months of the COVID-19 pandemic in the United Kingdom (UK) (late January to early April 2020), we received 754 URT samples from 425 symptomatic cases that tested positive for SARS-CoV-2 by RT-PCR targeting the RNA-dependent RNA polymerase (RdRp) gene [1] and that had a clear record of the dates of symptom onset and sample collection. These samples were collected as part of the First Few 100 surveillance study described in Boddington et al. [2]. Using RT-PCR cycle threshold (Ct) values as a semiquantitative measure of SARS-CoV-2 viral load identified that the level of SARS-CoV-2 RNA in the URT was greatest around symptom onset, steadily decreased during the first 10 days after illness onset and then plateaued (Figure 1). In the first week after symptom onset (days −2 to 7), geometric mean (GM) Ct was 28.18 (95% confidence interval (CI): 27.76–28.61). In the second week (days 8 to 14), GM Ct was 30.65 (95% CI: 29.82–31.52; p < 0.001 compared with week 1) and after 14 days, GM Ct was 31.60 (95% CI: 31.60–34.49; p = 0.01 compared with week 1). There was no significant difference in Ct values between days 8–14 and after 14 days (p = 0.49).
Ct: cycle threshold.
To capture the nonlinear relationship between days post symptom onset (days) and Ct value, a fractional polynomial model was used, indicating predictors days2 and days2ln(days). These were fitted in a random intercept regression model with ln(Ct value) as the outcome variable. Analysis accounted for multiple samples from the same individuals. The random intercept for individuals was not statistically significant, providing no evidence for dependencies within person, thus each individual sample was treated as being independent. The y axis was created in Stata 15.1 with reversed log scale*.
Continues here
https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.32.2001483
__
eof
__





Thank you for this quick explanation. How do supposedly intelligent people misunderstand? (Supposed, because they achieved doctoral-level recognition of their skills and knowledge.) I keep pointing out that the number of tests per person is multiple – using the Queensland government website as my go-to. (https://www.qld.gov.au/health/conditions/health-alerts/coronavirus-covid-19/current-status/statistics) Last reporting cycle: persons tested 1,911, samples tested 5,187. So, 3-4 tests have been required on each person’s sample for the experts to be able to come to a comfortably probable interpretation of whether the person was positive/negative for active COVID-19 at the time the sample was taken. [Apologies for any incorrect terminology. I’m an economic historian, not an epidemiologist, etc.!]
“This was written because some of you, perhaps some doing high-throughput testing of human specimens for the first time ever – need to take some time to learn about what it is you’re trying to achieve here.”
So how many labs have been doing it wrong and for how long? How many positive results have they reported and how many of those were false? Which labs didn’t RTFM before performing high-throughput testing? These are the real questions that aren’t being addressed here.
___
Recent Posts